Please support the World Food Safety Almanac by participating in our 1-minute reader survey.
Difference between revisions of "EuropeanUnion:European Union"
Tags: Mobile edit Mobile web edit |
m (Tramsen moved page European Union to European Union:European Union) |
(No difference)
| |
Revision as of 14:08, 22 November 2022
© worldfoodsafetyalmanac.bfr.berlin
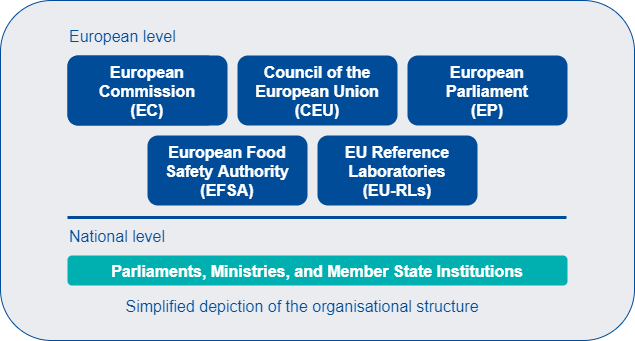
In 2000, the EU Commission's White Paper provided the impetus for a new food safety structure in Europe. A new concept for effective and comprehensive consumer health protection was introduced, whereby food safety is to be guaranteed in all stages of production and processing along the entire food chain (“from the farm to the fork”). In 2002, the European Food Safety Authority (EFSA) was established as an independent scientific body for risk assessment at the EU level. Following that, a network of competent institutions which operate as a link between the national and European levels, as well as between bodies in the various Member States, was created.
Regulations (EC) No. 178/2002 and the new Regulation (EU) 2019/1381 on the transparency and sustainability of the EU risk assessment in the food chain, which amends Regulation (EC) 178/2002, are the main legal foundations of food legislation in the European Union (EU).
In addition, Regulation (EU) 2017/625 which replaces Regulation (EC) 882/2004, lays down general principles of official controls performed to ensure compliance with food and feed law.
EU regulations apply directly in all Member States of the EU, without each one having to enact national laws.
Regulation (EC) No. 178/2002 lays down the general principles and requirements of food law within the EU. It covers all stages of food production and processing along the food chain, “from the farm to the fork”. In addition, it establishes and specifies the remits of EFSA and establishes the European Rapid Alert System for Food and Feed (RASFF). Regulation (EC) No. 882/2004 lays down the general principles of official controls performed to ensure compliance with food and feed law. This means that in the EU, in particular, the Member States are required to create a multi annual national control plan (MANCP) along with the corresponding reporting.
At the EU level, risk assessment and risk communication are formally separated from risk management. While risk assessments are undertaken by EFSA, EU risk management is dealt with by the European Commission (EC). Risk communication, the third element of risk analysis, is a shared responsibility between risk assessors and managers.
Regulation (EU) 2019/1381 on the transparency and sustainability of the EU risk assessment in the food chain – the “Transparency Regulation” – amends Regulation (EC) No. 178/2002, and has become applicable as of 27 March 2021. The aim of the Transparency Regulation is to increase the transparency of the EU risk assessment in the food chain, strengthen the quality and reliability of studies that EFSA bases its risk assessments on, revisit EFSA governance, achieve more coherent risk communication in the EU and improve the sustainability of EFSA’s expertise.
More information about the Transparency Regulation can be found at:
https://ec.europa.eu/food/safety/general_food_law/implementation-transparency-regulation_en
European Commission
| Name | European Commission |
|---|---|
| Acronym | EC |
| Activities |
|
| Responsibilities | |
| Location | Brussels, Belgium |
| URL | http://ec.europa.eu |
It is the role of the European Commission (EC) to promote the general interest of the EU as a whole. The “College of Commissioners” (currently one commissioner from each Member State) defines policy and makes decisions.
The EC is the EU's executive body: as the “guardian of the treaties”, the EC is responsible for ensuring that EU law is properly implemented and applied in all EU Member States. In the event of infringements or doubts about the interpretation on EU law, the European Court of Justice has the final say.
The EC is the only institution with the right of legislative initiative. In practice, the proposals for legislation (regulations, directives and decisions) are prepared by specific EC departments. The EC Directorate-General for Health and Food Safety (DG SANTE) prepares draft legislation in the area of food and feed as well as animal and plant health, for example, while the EC Directorate-General for Environment prepares draft legislation in that area.
Through the activities of Directorate F - Health and Food Audits and Analysis (Regulation (EC) No. 882/2004, Articles 45 and 46), which is part of the EC DG SANTE, the EC assesses how EU regulations related to food and feed safety, animal health, animal welfare, plant health and in the area of medical devices are implemented and enforced within the EU and in non EU countries that export to the EU. This is achieved mainly through inspections of the competent institutions of the Member States and non EU countries. The Directorate F - Directorate Health and Food Audits and Analysis provides information on the results of its assessments. Where appropriate, it also recommends measures to remedy shortcomings and monitors their implementation. Inspection reports are published on the Internet along with the comments by the countries inspected.
Council of the European Union
| Name | Council of the European Union |
|---|---|
| Acronym | CEU |
| Activities |
|
| Responsibilities | |
| Location | Brussels, Belgium |
| URL | http://www.consilium.europa.eu |
Together with the European Parliament, the Council of the European Union (CEU) is the main decision making body in the EU. One minister from each national government attends CEU meetings. Which minister attends a meeting depends on the topic to be discussed. The minister represents his/her Member State. Food safety issues, for example, are dealt with at meetings of the “Agriculture and Fisheries” CEU, while food labelling is addressed at the “Employment, Social Policy, Health and Consumer Affairs” CEU.
European legislation in the area of consumer health protection and food and feed safety is adopted through the “ordinary legislative procedure”, the most frequently applied legislative procedure in EU law. The “ordinary legislative procedure” means that draft legislation needs to be adopted by both the CEU and the European Parliament (EP).
European Parliament
| Name | European Parliament |
|---|---|
| Acronym | EP |
| Activities |
|
| Responsibilities | |
| Location | Strasbourg, France |
| URL | http://www.europarl.europa.eu |
The European Parliament (EP) is elected by the citizens of the EU. It has 705 members from 27 EU countries and represents the interests of about 500 million people. The fact that the EP is directly elected by the citizens helps guarantee the democratic legitimacy of European law.
The EP exercises democratic supervision over the other EU institutions, especially the EC. The EP has the power to approve or reject the nomination of commissioners and also has the right to censure the EC as a whole. The EP shares authority over the EU budget with the CEU and can therefore influence EU spending. At the end of the process, it either adopts or rejects the budget in its entirety.
The EP also prepares draft legislation in the area of food and feed safety. The EP has 20 standing committees and three subcommittees. The “Standing Committee on Environment, Public Health and Food Safety” deals with food safety and drinking water issues among others.
European Food Safety Authority
| Name | European Food Safety Authority |
|---|---|
| Acronym | EFSA |
| Activities |
|
| Responsibilities | |
| Location | Parma, Italy |
| URL | http://www.efsa.europa.eu |
European Food Safety Authority (EFSA) is an independent European Agency and the keystone of risk assessment regarding food and feed safety in the EU. In close collaboration with national institutions and in open consultation with other stakeholders, EFSA delivers independent scientific advice as well as clear and understandable communication on existing and emerging risks. In its capacity as a risk assessment authority, EFSA prepares scientific opinions and recommendations which form a sound foundation for European policies and legislation to support the EC, EP and EU Member States in taking effective and timely risk management decisions. All risk assessments are published on the EFSA website.
Although EFSA receives requests for risk assessments from the EC, EP and Member States, it also undertakes scientific work on its own initiative. EFSA's remit covers risk assessments on food and feed safety, nutrition, animal health and welfare, plant protection and plant health.
European Union Reference Laboratories
| Name | European Union Reference Laboratories |
|---|---|
| Acronym | EU-RLs |
| Activities |
|
European Union Reference Laboratories (EU-RLs) are analytical laboratories designated by EU directives and regulations. Several EU-RLs are part of the Joint Research Centre (JRC), which is a Directorate General of the EC. EU-RLs provide the EC with technical and scientific support in the area of diagnostic and analytical tests. The remit of EU-RLs includes the set up of EU wide test standards, routine procedures and reliable methods, the organisation of comparative tests, training of analysts from national laboratories and networking with National Reference Laboratories. EU-RLs have an overview of international standards and practices, reference substances, reagents and their suppliers.
The EU-RLs for food and feed are listed in Annex VII of Regulation (EC) No. 882/2004: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:165:0001:0141:EN:PDF